Get in touch
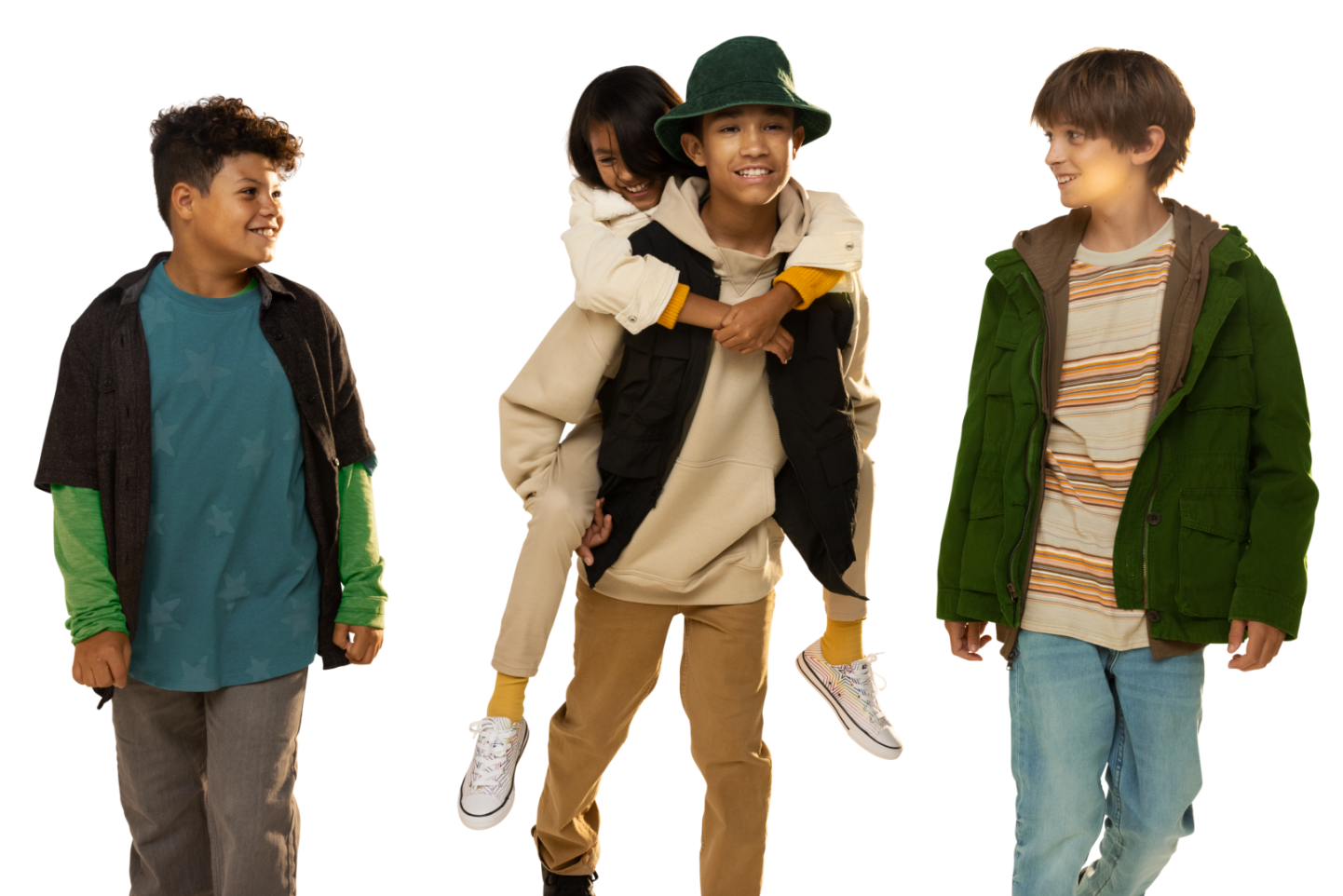
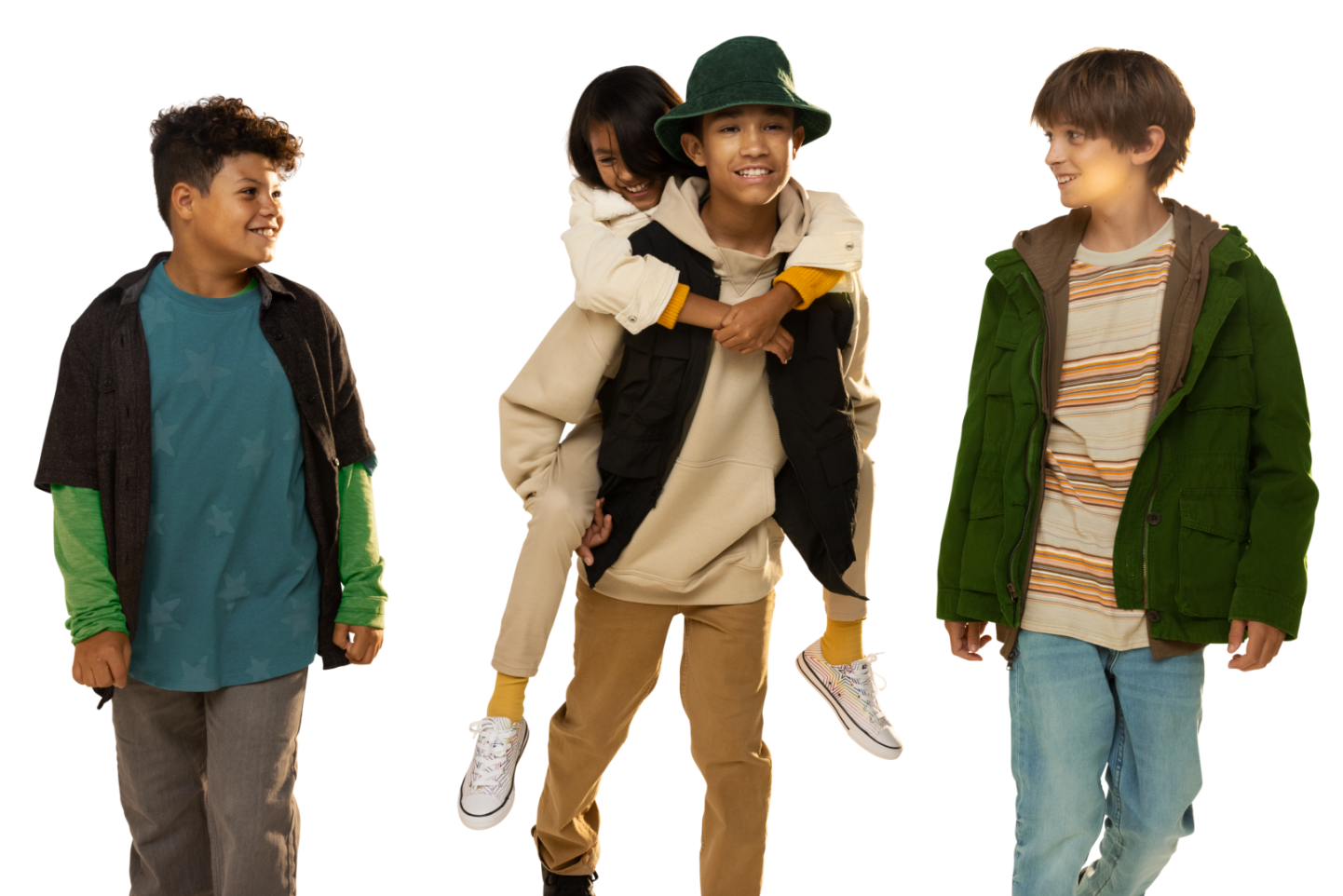
Drupal\Component\Utility\Html::getClass() (Line: 86)
__TwigTemplate_9817c38a0908243ba0944aded5477150->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 227)
Drupal\Core\Render\Renderer->render() (Line: 491)
Drupal\Core\Template\TwigExtension->escapeFilter() (Line: 57)
__TwigTemplate_124c5fe419b4a4997fef2308a0efac7b->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 227)
Drupal\Core\Render\Renderer->render() (Line: 491)
Drupal\Core\Template\TwigExtension->escapeFilter() (Line: 105)
__TwigTemplate_8ad3fc6d4883d1b76c9edd5dc3cfb2a7->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 227)
Drupal\Core\Render\Renderer->render() (Line: 242)
Drupal\Core\Render\MainContent\HtmlRenderer->Drupal\Core\Render\MainContent\{closure}() (Line: 627)
Drupal\Core\Render\Renderer->executeInRenderContext() (Line: 235)
Drupal\Core\Render\MainContent\HtmlRenderer->prepare() (Line: 131)
Drupal\Core\Render\MainContent\HtmlRenderer->renderResponse() (Line: 90)
Drupal\Core\EventSubscriber\MainContentViewSubscriber->onViewRenderArray() (Line: 246)
Symfony\Component\EventDispatcher\EventDispatcher::Symfony\Component\EventDispatcher\{closure}() (Line: 206)
Symfony\Component\EventDispatcher\EventDispatcher->callListeners() (Line: 56)
Symfony\Component\EventDispatcher\EventDispatcher->dispatch() (Line: 188)
Symfony\Component\HttpKernel\HttpKernel->handleRaw() (Line: 76)
Symfony\Component\HttpKernel\HttpKernel->handle() (Line: 53)
Drupal\Core\StackMiddleware\Session->handle() (Line: 48)
Drupal\Core\StackMiddleware\KernelPreHandle->handle() (Line: 28)
Drupal\Core\StackMiddleware\ContentLength->handle() (Line: 201)
Drupal\page_cache\StackMiddleware\PageCache->fetch() (Line: 138)
Drupal\page_cache\StackMiddleware\PageCache->lookup() (Line: 87)
Drupal\page_cache\StackMiddleware\PageCache->handle() (Line: 50)
Drupal\ban\BanMiddleware->handle() (Line: 48)
Drupal\Core\StackMiddleware\ReverseProxyMiddleware->handle() (Line: 51)
Drupal\Core\StackMiddleware\NegotiationMiddleware->handle() (Line: 53)
Drupal\Core\StackMiddleware\AjaxPageState->handle() (Line: 51)
Drupal\Core\StackMiddleware\StackedHttpKernel->handle() (Line: 715)
Drupal\Core\DrupalKernel->handle() (Line: 19)
Drupal\Component\Utility\Html::getClass() (Line: 90)
__TwigTemplate_8009544750248676a05f5dd0de2ddd91->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 227)
Drupal\Core\Render\Renderer->render() (Line: 491)
Drupal\Core\Template\TwigExtension->escapeFilter() (Line: 57)
__TwigTemplate_124c5fe419b4a4997fef2308a0efac7b->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 227)
Drupal\Core\Render\Renderer->render() (Line: 491)
Drupal\Core\Template\TwigExtension->escapeFilter() (Line: 105)
__TwigTemplate_8ad3fc6d4883d1b76c9edd5dc3cfb2a7->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 227)
Drupal\Core\Render\Renderer->render() (Line: 242)
Drupal\Core\Render\MainContent\HtmlRenderer->Drupal\Core\Render\MainContent\{closure}() (Line: 627)
Drupal\Core\Render\Renderer->executeInRenderContext() (Line: 235)
Drupal\Core\Render\MainContent\HtmlRenderer->prepare() (Line: 131)
Drupal\Core\Render\MainContent\HtmlRenderer->renderResponse() (Line: 90)
Drupal\Core\EventSubscriber\MainContentViewSubscriber->onViewRenderArray() (Line: 246)
Symfony\Component\EventDispatcher\EventDispatcher::Symfony\Component\EventDispatcher\{closure}() (Line: 206)
Symfony\Component\EventDispatcher\EventDispatcher->callListeners() (Line: 56)
Symfony\Component\EventDispatcher\EventDispatcher->dispatch() (Line: 188)
Symfony\Component\HttpKernel\HttpKernel->handleRaw() (Line: 76)
Symfony\Component\HttpKernel\HttpKernel->handle() (Line: 53)
Drupal\Core\StackMiddleware\Session->handle() (Line: 48)
Drupal\Core\StackMiddleware\KernelPreHandle->handle() (Line: 28)
Drupal\Core\StackMiddleware\ContentLength->handle() (Line: 201)
Drupal\page_cache\StackMiddleware\PageCache->fetch() (Line: 138)
Drupal\page_cache\StackMiddleware\PageCache->lookup() (Line: 87)
Drupal\page_cache\StackMiddleware\PageCache->handle() (Line: 50)
Drupal\ban\BanMiddleware->handle() (Line: 48)
Drupal\Core\StackMiddleware\ReverseProxyMiddleware->handle() (Line: 51)
Drupal\Core\StackMiddleware\NegotiationMiddleware->handle() (Line: 53)
Drupal\Core\StackMiddleware\AjaxPageState->handle() (Line: 51)
Drupal\Core\StackMiddleware\StackedHttpKernel->handle() (Line: 715)
Drupal\Core\DrupalKernel->handle() (Line: 19)
Drupal\Component\Utility\Html::getClass() (Line: 86)
__TwigTemplate_9817c38a0908243ba0944aded5477150->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 227)
Drupal\Core\Render\Renderer->render() (Line: 491)
Drupal\Core\Template\TwigExtension->escapeFilter() (Line: 59)
__TwigTemplate_e961ea1c8e853118d5cc3187eff632ef->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 227)
Drupal\Core\Render\Renderer->render() (Line: 491)
Drupal\Core\Template\TwigExtension->escapeFilter() (Line: 94)
__TwigTemplate_85fefaff580ea00b443ee6f44832f69e->block_content() (Line: 446)
Twig\Template->yieldBlock() (Line: 73)
__TwigTemplate_85fefaff580ea00b443ee6f44832f69e->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 250)
Drupal\Core\Render\Renderer->doRenderRoot() (Line: 141)
Drupal\Core\Render\Renderer->Drupal\Core\Render\{closure}() (Line: 627)
Drupal\Core\Render\Renderer->executeInRenderContext() (Line: 140)
Drupal\Core\Render\Renderer->renderInIsolation() (Line: 167)
Drupal\Core\Render\Renderer->doRenderPlaceholder() (Line: 729)
Drupal\Core\Render\Renderer->Drupal\Core\Render\{closure}()
Fiber->start() (Line: 737)
Drupal\Core\Render\Renderer->replacePlaceholders() (Line: 262)
Drupal\Core\Render\Renderer->doRenderRoot() (Line: 141)
Drupal\Core\Render\Renderer->Drupal\Core\Render\{closure}() (Line: 627)
Drupal\Core\Render\Renderer->executeInRenderContext() (Line: 140)
Drupal\Core\Render\Renderer->renderInIsolation() (Line: 111)
Drupal\Core\Render\Renderer->renderRoot() (Line: 253)
Drupal\Core\Render\HtmlResponseAttachmentsProcessor->renderPlaceholders() (Line: 93)
Drupal\Core\Render\HtmlResponseAttachmentsProcessor->processAttachments() (Line: 45)
Drupal\Core\EventSubscriber\HtmlResponseSubscriber->onRespond() (Line: 246)
Symfony\Component\EventDispatcher\EventDispatcher::Symfony\Component\EventDispatcher\{closure}() (Line: 206)
Symfony\Component\EventDispatcher\EventDispatcher->callListeners() (Line: 56)
Symfony\Component\EventDispatcher\EventDispatcher->dispatch() (Line: 216)
Symfony\Component\HttpKernel\HttpKernel->filterResponse() (Line: 204)
Symfony\Component\HttpKernel\HttpKernel->handleRaw() (Line: 76)
Symfony\Component\HttpKernel\HttpKernel->handle() (Line: 53)
Drupal\Core\StackMiddleware\Session->handle() (Line: 48)
Drupal\Core\StackMiddleware\KernelPreHandle->handle() (Line: 28)
Drupal\Core\StackMiddleware\ContentLength->handle() (Line: 201)
Drupal\page_cache\StackMiddleware\PageCache->fetch() (Line: 138)
Drupal\page_cache\StackMiddleware\PageCache->lookup() (Line: 87)
Drupal\page_cache\StackMiddleware\PageCache->handle() (Line: 50)
Drupal\ban\BanMiddleware->handle() (Line: 48)
Drupal\Core\StackMiddleware\ReverseProxyMiddleware->handle() (Line: 51)
Drupal\Core\StackMiddleware\NegotiationMiddleware->handle() (Line: 53)
Drupal\Core\StackMiddleware\AjaxPageState->handle() (Line: 51)
Drupal\Core\StackMiddleware\StackedHttpKernel->handle() (Line: 715)
Drupal\Core\DrupalKernel->handle() (Line: 19)
Drupal\Component\Utility\Html::getClass() (Line: 86)
__TwigTemplate_9817c38a0908243ba0944aded5477150->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 227)
Drupal\Core\Render\Renderer->render() (Line: 491)
Drupal\Core\Template\TwigExtension->escapeFilter() (Line: 75)
__TwigTemplate_e961ea1c8e853118d5cc3187eff632ef->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 510)
Drupal\Core\Render\Renderer->doRender() (Line: 227)
Drupal\Core\Render\Renderer->render() (Line: 491)
Drupal\Core\Template\TwigExtension->escapeFilter() (Line: 94)
__TwigTemplate_85fefaff580ea00b443ee6f44832f69e->block_content() (Line: 446)
Twig\Template->yieldBlock() (Line: 73)
__TwigTemplate_85fefaff580ea00b443ee6f44832f69e->doDisplay() (Line: 402)
Twig\Template->yield() (Line: 358)
Twig\Template->display() (Line: 373)
Twig\Template->render() (Line: 51)
Twig\TemplateWrapper->render() (Line: 34)
twig_render_template() (Line: 380)
Drupal\Core\Theme\ThemeManager->render() (Line: 497)
Drupal\Core\Render\Renderer->doRender() (Line: 250)
Drupal\Core\Render\Renderer->doRenderRoot() (Line: 141)
Drupal\Core\Render\Renderer->Drupal\Core\Render\{closure}() (Line: 627)
Drupal\Core\Render\Renderer->executeInRenderContext() (Line: 140)
Drupal\Core\Render\Renderer->renderInIsolation() (Line: 167)
Drupal\Core\Render\Renderer->doRenderPlaceholder() (Line: 729)
Drupal\Core\Render\Renderer->Drupal\Core\Render\{closure}()
Fiber->start() (Line: 737)
Drupal\Core\Render\Renderer->replacePlaceholders() (Line: 262)
Drupal\Core\Render\Renderer->doRenderRoot() (Line: 141)
Drupal\Core\Render\Renderer->Drupal\Core\Render\{closure}() (Line: 627)
Drupal\Core\Render\Renderer->executeInRenderContext() (Line: 140)
Drupal\Core\Render\Renderer->renderInIsolation() (Line: 111)
Drupal\Core\Render\Renderer->renderRoot() (Line: 253)
Drupal\Core\Render\HtmlResponseAttachmentsProcessor->renderPlaceholders() (Line: 93)
Drupal\Core\Render\HtmlResponseAttachmentsProcessor->processAttachments() (Line: 45)
Drupal\Core\EventSubscriber\HtmlResponseSubscriber->onRespond() (Line: 246)
Symfony\Component\EventDispatcher\EventDispatcher::Symfony\Component\EventDispatcher\{closure}() (Line: 206)
Symfony\Component\EventDispatcher\EventDispatcher->callListeners() (Line: 56)
Symfony\Component\EventDispatcher\EventDispatcher->dispatch() (Line: 216)
Symfony\Component\HttpKernel\HttpKernel->filterResponse() (Line: 204)
Symfony\Component\HttpKernel\HttpKernel->handleRaw() (Line: 76)
Symfony\Component\HttpKernel\HttpKernel->handle() (Line: 53)
Drupal\Core\StackMiddleware\Session->handle() (Line: 48)
Drupal\Core\StackMiddleware\KernelPreHandle->handle() (Line: 28)
Drupal\Core\StackMiddleware\ContentLength->handle() (Line: 201)
Drupal\page_cache\StackMiddleware\PageCache->fetch() (Line: 138)
Drupal\page_cache\StackMiddleware\PageCache->lookup() (Line: 87)
Drupal\page_cache\StackMiddleware\PageCache->handle() (Line: 50)
Drupal\ban\BanMiddleware->handle() (Line: 48)
Drupal\Core\StackMiddleware\ReverseProxyMiddleware->handle() (Line: 51)
Drupal\Core\StackMiddleware\NegotiationMiddleware->handle() (Line: 53)
Drupal\Core\StackMiddleware\AjaxPageState->handle() (Line: 51)
Drupal\Core\StackMiddleware\StackedHttpKernel->handle() (Line: 715)
Drupal\Core\DrupalKernel->handle() (Line: 19)
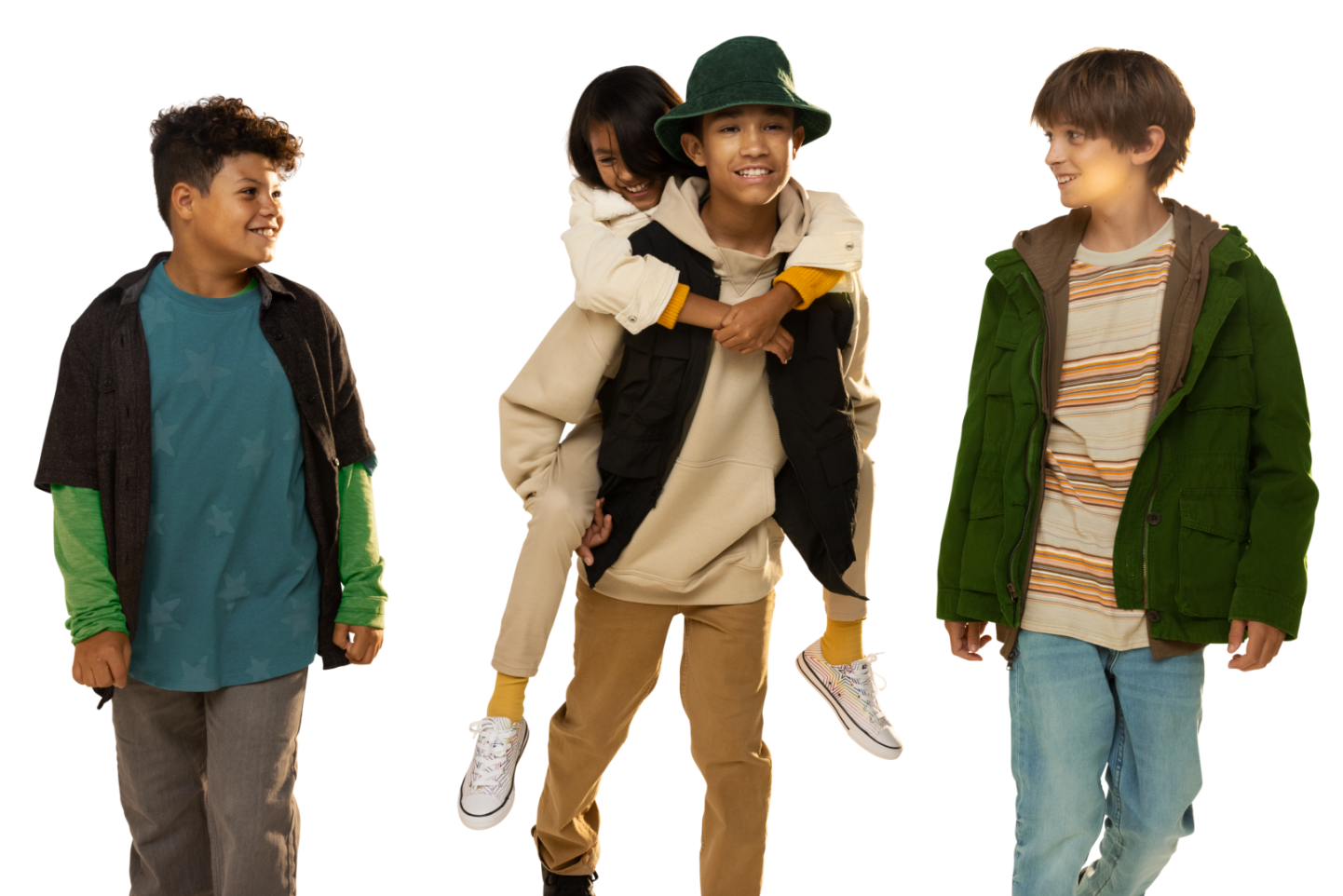
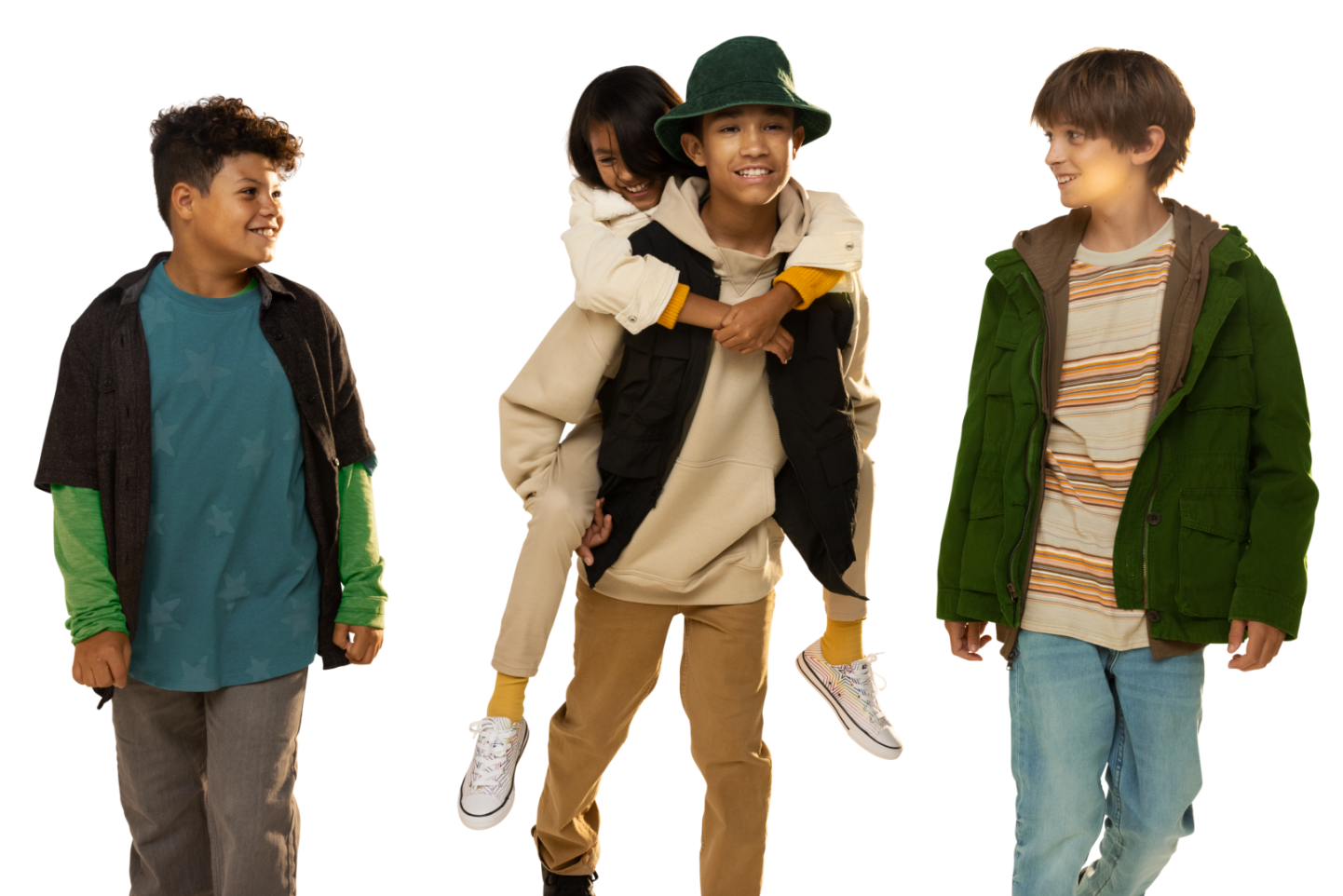
Verkazia® (cyclosporine ophthalmic emulsion) 0.1% is a calcineurin inhibitor immunosuppressant indicated for the treatment of vernal keratoconjunctivitis in children and adults.
Potential for eye injury and contamination: To avoid the potential for eye injury and contamination, advise patient not to touch the vial tip to the eye or other surfaces.
The most common adverse reactions reported in greater than 5% of patients were eye pain (12%) and eye pruritus (8%), which were usually transitory and occurred during instillation.
You are encouraged to report adverse reactions of prescription drugs to FDA at 1-800-FDA-1088. You may also report side effects to Harrow at 1-833-4HARROW (1-833-442-7769).
Please see Full Prescribing Information.
REFERENCES: 1. Verkazia [package insert]. Emeryville, CA: Santen Inc.; 2021. 2. Leonardi A, Doan S, Amrane M, et al; for VEKTIS Study Group. A randomized, controlled trial of cyclosporine A cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. 2019;126(5):671-681. doi:10.1016/j.ophtha.2018.12.027. 3. Bremond-Gignac D, Doan S, Amrane M, et al; for VEKTIS Study Group. Twelve-month results of cyclosporine A cationic emulsion in a randomized study in patients with pediatric vernal keratoconjunctivitis. Am J Ophthalmol. 2020;212:116-126. doi:10.1016/j.ajo.2019.11.020.
REFERENCES: 1. Verkazia [package insert]. Emeryville, CA: Santen Inc.; 2021. 2. Leonardi A, Doan S, Amrane M, et al; for VEKTIS Study Group. A randomized, controlled trial of cyclosporine A cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. 2019;126(5):671-681. doi:10.1016/j.ophtha.2018.12.027. 3. Lallemand F, Daull P, Benita S, Buggage R, Garrigue J-S. Successfully improving ocular drug delivery using the cationic nanoemulsion, Novasorb. J Drug Deliv. 2012;604204. doi:10.1155/2012/604204. 4. Dutescu R M, Panfil C, Merkel O M, et. Al. Semiflourinated alkanes as a liquid drug carrier system for topical ocular drug delivery. European Journal of Pharmaceutics and biopharmaceutics. 5. Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye (Lond). 2004;18(4):345-351. doi:10.1038/sj.eye.6700675. 6. Research and Markets. Vernal keratoconjunctivitis—epidemiology forecast—2030. https://www.researchandmarkets.com/reports/5118879/vernal-keratoconjunctivitis-epidemiology. Accessed October 17, 2021. 7. Vichyanond P, Pacharn P, Pleyer U, Leonardi A. Vernal keratoconjunctivitis: a severe allergic eye disease with remodeling changes. Pediatr Allergy Immunol. 2014;25(4):314-322. doi:10.1111/pai.12197. 8. Masters JS, Bunya VY, Woodward MA, Halfpenny C. Vernal keratoconjunctivitis. American Academy of Ophthalmology Eye Wiki. https://eyewiki.aao.org/Vernal_Keratoconjunctivitis. Updated September 5, 2021. Accessed October 17, 2021. 9. Ang M, Ti S-E, Loh R, et al. Steroid-induced ocular hypertension in Asian children with severe vernal keratoconjunctivitis. Clin Ophthalmol. 2012;6:1253-1258. doi:10.2147/OPTH.S32936. 10. Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. 2013;2(2):73-88. doi:10.1007/s40123-013-0019-y.
REFERENCES: 1. Verkazia [package insert]. Emeryville, CA: Santen Inc.; 2021. 2. Leonardi A, Doan S, Amrane M, et al; for VEKTIS Study Group. A randomized, controlled trial of cyclosporine A cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. 2019;126(5):671-681. doi:10.1016/j.ophtha.2018.12.027. 3. Data on file. Santen Inc. 2021. 4. Charters L. FDA approves cyclosporine A eye drop for vernal keratoconjunctivitis. Ophthalmol Times. 2021;46(12):8-9. https://www.ophthalmologytimes.com/view/fda-approves-cyclosporine-a-drop-for-vernal-keratoconjunctivitis. Published August 8, 2021. Accessed October 18, 2021.
REFERENCES: 1. Verkazia [package insert]. Emeryville, CA: Santen Inc.; 2021. 2. Bremond-Gignac D, Doan S, Amrane M, et al; for VEKTIS Study Group. Twelve-month results of cyclosporine A cationic emulsion in a randomized study in patients with pediatric vernal keratoconjunctivitis. Am J Ophthalmol. 2020;212:116-126. doi:10.1016/j.ajo.2019.11.020.
REFERENCES: 1. Verkazia [package insert]. Emeryville, CA: Santen Inc.; 2021. 2. Vichyanond P, Pacharn P, Pleyer U, Leonardi A. Vernal keratoconjunctivitis: a severe allergic eye disease with remodeling changes. Pediatr Allergy Immunol. 2014;25(4):314-322. doi:10.1111/pai.12197. 3. Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye (Lond). 2004;18(4):345-351. doi:10.1038/sj.eye.6700675.
Verkazia® (cyclosporine ophthalmic emulsion) 0.1% is an eye drop that helps control the ongoing inflammation of vernal keratoconjunctivitis (VKC). VKC is an allergic condition where the body’s immune system is overactive and causes inflammation in the eye.
Be careful not to touch the vial tip to your eye or other surfaces, to help avoid potential eye injury or contamination of the product.
Contact lenses should be removed before applying Verkazia and may be reinserted 15 minutes after instillation.
The most common side effect is a temporary burning sensation. Other side effects include eye redness, eye discomfort, itchiness, and foreign body sensation.
You are encouraged to report adverse reactions of prescription drugs to FDA at 1-800-FDA-1088. You may also report side effects to Harrow at 1-833-4HARROW (1-833-442-7769).
Click here to see the full Product Information.